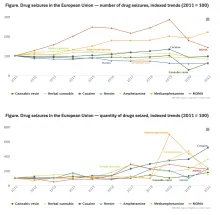
Methylenedioxymethamphetamine (MDMA or 'Ecstasy') drug profile
MDMA ('Ecstasy') drug profile
MDMA is a synthetic substance commonly known as ecstasy, although the latter term has now been generalised to cover a wide range of other substances. Originally developed in 1912 by the Merck chemical company, it was never marketed as such. Although proposed as an aid to psychiatric counselling, therapeutic use is extremely limited. Illicit MDMA is normally seen as tablets, many of which are manufactured in Europe. It acts as a central nervous system (CNS) stimulant and has a weak hallucinogenic property more accurately described as increased sensory awareness. MDMA is under international control.
Chemistry
Molecular structure

Molecular formula: C11H15NO2
Molecular weight: 193.2
MDMA is an abbreviation for methylenedioxy-methylamphetamine. The formal (IUPAC) name is N-methyl-1-(3,4-methylenedioxyphenyl)propan-2-amine, but MDMA (CAS-42542-10-09) is commonly known as 3,4-methylenedioxymethamphetamine or methylenedioxy-methylamfetamine. Other chemical names include N,α-dimethyl-3,4-methylenedioxyphenethylamine or, less usually, N-methyl-1-(1,3-benzodioxol-5-yl)-2-propanamine. MDMA is a member of the larger group of ring-substituted phenethylamines. As with other phenethylamines, and like its close relative methamphetamine, MDMA also exists in two enantiomeric forms (R and S).
Physical form
The most common salt is the hydrochloride (CAS-64057-70-1) which occurs as a white or off-white powder or as crystals soluble in water. The phosphate salt is also encountered. Illicit products are seen principally as white tablets with a characteristic impression (logo), less commonly as white powders or capsules. MDMA base is a colourless oil insoluble in water.
Pharmacology
Whereas phenethylamines without ring substitution usually behave as stimulants, ring substitution (as in MDMA) leads to a modification in the pharmacological properties. Ingestion of MDMA causes euphoria, increased sensory awareness and mild central stimulation. It is less hallucinogenic than its lower homologue, methylenedioxyamphetamine (MDA). The terms empathogenic and entactogenic have been coined to describe the socialising effects of MDMA. Following ingestion, most of the dose of MDMA is excreted in the urine unchanged. Major metabolites are 3,4-methylenedioxyamphetamine (MDA) and O-demethylated compounds. Following a dose of 75 mg, the maximum plasma concentration of around 0.13 mg/L is reached within two hours. The plasma half-life is 6–7 hours. In animals, MDMA causes neurotoxicity, as evidenced by anatomical changes in axon structure and a persisting reduction in brain serotonin levels. The significance of these findings to human users is still unclear, although cognitive impairment is associated with MDMA use. Some of the pharmacodynamic and toxic effects of MDMA vary, depending on which enantiomer is used. However, almost all illicit MDMA exists as a racemic mixture. Fatalities following a dose of 300 mg have been noted, but toxicity depends on many factors, including individual susceptibility and the circumstances in which MDMA is used.
Synthesis and precursors
There are four principal precursors which can be used in the manufacture of MDMA and related drugs: safrole, isosafrole, piperonal and 3,4-methylenedioxyphenyl-2-propanone (PMK). Safrole is the key starting material in so far as the other three can be synthesised from it. In the original Merck patent of 1914, safrole was reacted with hydrobromic acid to form bromosafrole, which was converted to MDMA using methylamine. Many illicit syntheses start with PMK and use either the Leuckart route or various reductive aminations including the aluminium foil method. All of these methods produce racemic MDMA. The four precursors noted above are listed in Table I of the United Nations 1988 Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. The corresponding EU legislation is set out in Council Regulation (EEC) No 3677/90 (as later amended), which governs trade between the EU and third countries.
Mode of use
MDMA in tablet form is almost always used orally (ingested), but the powdered form could also be snorted, inhaled or injected, although the latter route is rarely observed in the context of recreational ecstasy use.
Other names
As some of the above names suggest, MDMA is a derivative of amphetamine and a member of the phenethylamine family. A number of homologous compounds with broadly similar effects, e.g. MDA (methylenedioxyamphetamine), MDEA (methylenedioxyethylamphetamine) and MBDB (N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine), have appeared, but have proved less popular. These and many other more distant relatives of MDMA have now been subsumed by the generic term ecstasy. Street terms for MDMA include Adam and XTC, but often reflect the imprinted logo, e.g. Mitsubishis, Love Doves and many others.
Analysis
In common with many of its homologues, MDMA reacts with the Marquis field test to produce a dark blue/black coloration. The mass spectrum shows limited structure with a major ion at m/z = 58 and other ions at m/z = 135 and 77. Using gas chromatography, the limits of detection in plasma and urine are 1.6 μg/L and 47 μg/L respectively.
Control status
MDMA, shown as (+/–)-N,α-dimethyl-3,4-(methylene-dioxy)phenethylamine, is listed in Schedule I of the United Nations 1971 Convention on Psychotropic Substances.
Medical use
MDMA once found limited use in psychiatric counselling, but its therapeutic use is now rare.
Publications
Infographics and media
Bibliography
Iversen, L. (2006), Speed, Ecstasy, Ritalin: the science of amphetamines, Oxford University Press, Oxford.
King, L. A. and McDermott, S. (2004), ‘Drugs of abuse’, in: Moffat, A. C., Osselton, M. D. and Widdop, B. (eds.) (2004), Clarke's analysis of drugs and poisons, 3rd edn, Vol. 1, pp. 37–52, Pharmaceutical Press, London.
Moffat, A. C., Osselton, M, D. and Widdop, B, (eds.) (2004), Clarke's analysis of drugs and poisons, 3rd edn, Vol. 2, Pharmaceutical Press, London.
Shulgin, A and Shulgin, A, (1992), PIHKAL: A chemical love story, Transform Press, Berkeley, CA.
United Nations (2006), Multilingual Dictionary of Narcotic Drugs and Psychotropic Substances under International Control, United Nations, New York.
United Nations (2006), Recommended Methods for the Identification and analysis of amphetamine, methamphetamine and their ring-substituted analogues in seized materials (revised and updated), Manual for Use by National Drug Testing Laboratories, United Nations, New York.
United Nations Office on Drugs and Crime (2003), Ecstasy and Amphetamines Global Survey 2003, United Nations Office on Drugs and Crime, Vienna (http://www.unodc.org/pdf/publications/report_ats_2003-09-23_1.pdf).
United Nations Office on Drugs and Crime (2004), World Drug Report 2004, Vol. 1: Analysis, United Nations Office on Drugs and Crime, Vienna (http://www.unodc.org/pdf/WDR_2004/volume_1.pdf).