Increasing access to hepatitis C care through drug services
Elimination of viral hepatitis as a public health threat
The introduction of direct-acting antiviral (DAA) therapy has profoundly changed and improved the clinical management of hepatitis C virus (HCV) infection and given impetus to the WHO goal of eliminating HCV infection as a public health threat by 2030. Although DAA therapy has provided the tools to achieve this, HCV elimination is contingent on substantially increasing the number of people tested, diagnosed, linked to care and treated. Improving HCV care among marginalised high-risk populations — such as people who inject drugs — is paramount in these efforts.
The European Union and all its Member States are committed to the Sustainable Development Goals and most countries have adopted policies to achieve the elimination of viral hepatitis as a public health threat by 2030.
European clinical guidelines (EASL and WHO) recommend that all patients with chronic liver disease as a result of HCV infection should be considered for therapy, regardless of disease stage. Furthermore, they recommend that treatment be provided to individuals at risk of transmitting the disease, including people currently injecting drugs. The beneficial impact of hepatitis C treatment on the infected individual and its indirect impact on reducing onward transmission in the community make 'testing and linkage to treatment' a core component of the hepatitis elimination strategy.
The EU Drugs Strategy 2013-2020 calls on Member States to invest in research to achieve a substantial reduction of viral hepatitis, and the current 2017-2020 Action Plan on Drugs includes the objective of the scaling up of coverage and improving access of people who inject drugs (PWID) to relevant prevention and harm reduction measures.
EMCDDA Harm Reduction initiative
Supporting the HCV elimination agenda – an EMCDDA Initiative to increase access to testing and treatment through drugs services
Due to the importance of PWID as a key population for the elimination of hepatitis C in Europe, increasing their access to HCV testing and care is a goal in European and national hepatitis C policies. Despite this, HCV testing remains low among PWID and effective approaches to promote testing as the first element of a cascade of care are particularly needed.
As HCV treatment is now highly effective, the main priority and challenge is to efficiently identify infected individuals to treat. Therefore this EMCDDA Harm Reduction initiative is focusing on hepatitis C testing. It aims to support countries in reaching the elimination goal by increasing access of PWID to hepatitis care through drugs services.
Building on the framework for response development and implementation presented in the EMCDDA publication Health and social responses to drug problems: a European guide (2017), a set of new materials and tools are being developed under this initiative, which correspond to the three stages: problem definition, response selection and implementation, as illustrated in Figure 1 below.
Figure 1. EMCDDA Harm Reduction initiative: Increasing access to HCV testing and care through drugs services

The materials can be used individually or in combination. For more details and to access the available materials see below.
Spotlight: What is an EMCDDA Harm Reduction initiative?
Under its Strategy 2025, the EMCDDA has defined ambitious goals regarding improving its customer orientation and services. To this end, the agency developed a holistic conceptual model for identifying and planning responses and extends its Best Practice Portal to include the identification of effective examples of programme implementation and models of care. In 2018, the EMCDDA furthermore started to develop a set of support tools for selected response areas in particular in the agency’s public health priority areas: the prevention and control of drug-related infectious diseases; and the reduction of morbidity and mortality related to the use of drugs. An EMCDDA harm reduction 'initiative' is the term used to describe a coherent package of service- and output-oriented activities, that pulls together and makes use of the agency’s ongoing monitoring activities in the field of epidemiology and responses, as well as existing evidence-based intervention guidance, in order to develop high-quality materials for capacity building and training activities for those working in the field. The initiatives aim to produce structured tools in support of EU Member States’ efforts to improve national practices, namely: tools to assess the situation and needs in the two fields; to identify current barriers and facilitators to evidence-based action; and to plan and implement effective responses.
Assessing barriers and opportunities
Assessing barriers and opportunities for increasing PWID’s access to HCV testing and care through drugs services
Increasing access to HCV testing in drug services is the starting point for the EMCDDA initiative. The (diagnostic) process of identifying barriers and opportunities to HCV testing in drugs services involves:
- Mapping and understanding the current situation regarding hepatitis C elimination among PWID and the available testing and treatment responses, using the EMCDDA elimination barometer;
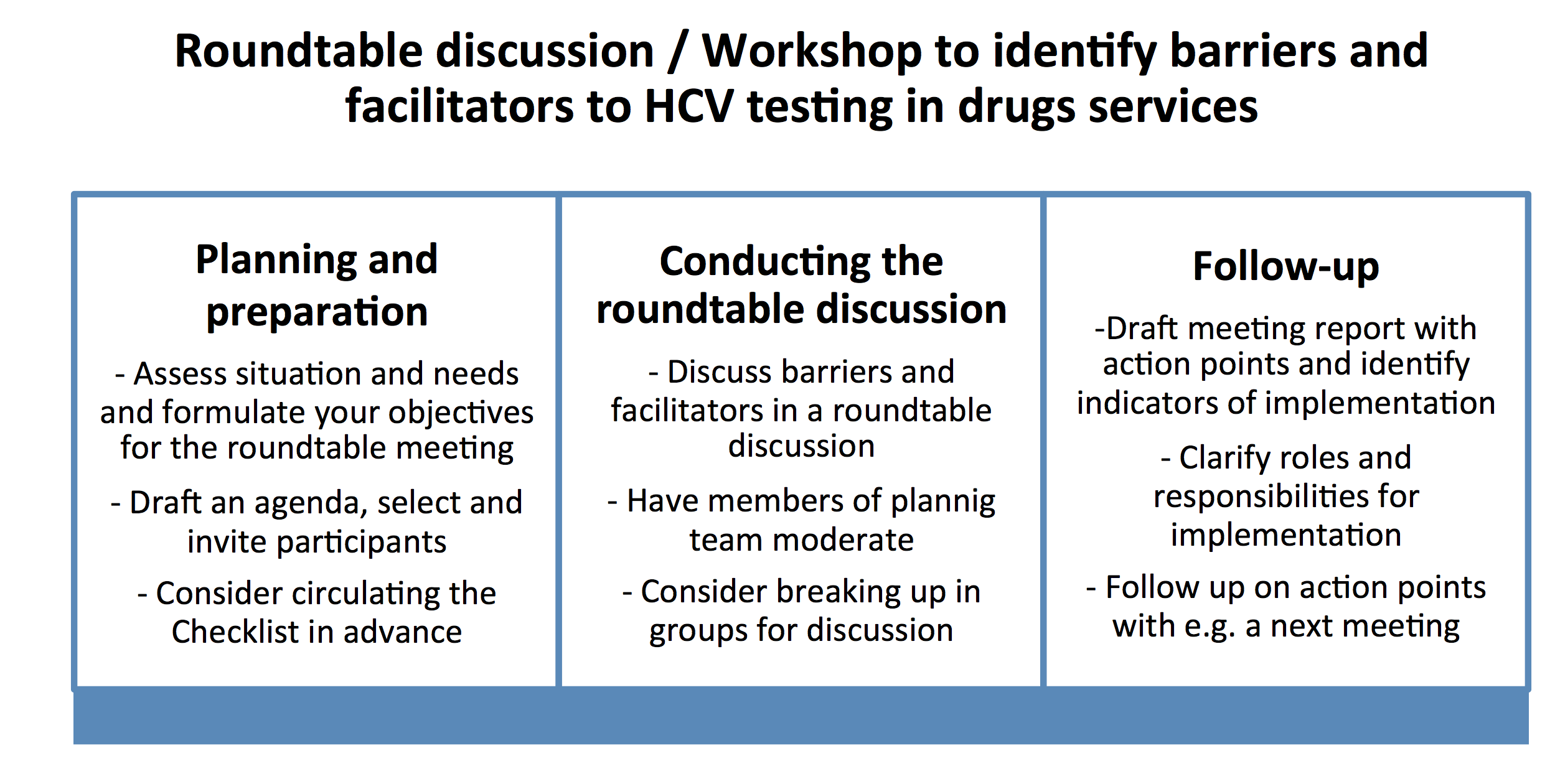
- Conducting a roundtable discussion or workshop with main stakeholders at national (or local) level using a checklist of barriers and facilitators to HCV testing; and,
- Developing an action plan, including a process for monitoring and evaluating the implementation of the actions.
The diagnostic process is described in a practical manual which will be published towards the end of 2019.
EMCDDA Elimination Barometer
An essential preparatory step for assessing barriers and facilitators to HCV testing in drugs services is mapping and understanding the current epidemiological context and response situation. This helps to obtain clarity about the context in which any further activities are taking place, is an important input to discussions with stakeholders and provides the basis for choosing the appropriate objectives. Knowing the size of the PWID target population and of the HCV epidemic, and being aware of the current infrastructure for HCV testing and treatment in your country is important background knowledge for identifying the need for and barriers to HCV testing, and to define or ‘diagnose’ whether there is a problem.
The EMCDDA elimination barometer is a new tool that can support this diagnosis. It is composed of indicators routinely reported to the agency which can assist the EU and Member States to assess progress towards the elimination of HBV and HCV infection, based on the WHO evaluation framework for viral hepatitis. It includes, for example, information about the size of the HCV epidemic among people who inject drugs, on the national policy context, and on the scale of prevention measures in place in a country. These indicators help to assess progress towards the elimination of HBV and HCV infection at national level and to identify data gaps. The five building blocks of the elimination barometer are presented in Figure 2 below. Each building block includes quantitative and/or qualitative indicators with a corresponding target. Showing the data at the start of the roundtable discussion/workshop (see section below) provides a basis to stimulate the discussion, helping to assess needs in the implementation of prevention and treatment responses as well as identifying data gaps. The full set of 30 country barometers is under preparation and will be launched in October 2019.
Figure 2. Elimination of viral hepatitis among PWID - EMCDDA indicators and related 2020 WHO elimination targets

Checklist to identify facilitators and barriers to testing
The next step in the diagnostic process includes consideration of the Checklist of barriers and facilitators to testing ´Overcoming barriers to HCV testing in drug treatment services for PWID´. The checklist can be used as a preparatory step for the roundtable discussion or workshop, or can be used to structure the discussions themselves. The checklist is constructed to help identify what main barriers prevent implementation of HCV testing and care in drugs services. The questions are structured into three levels of barriers: system, provider, and client level. Barriers are then organised into sub-categories for each of the three levels. Preparatory work on the checklist was presented as poster at INHSU 2018 and the final version will be launched together with the Manual towards the end of 2019.
Roundtable discussion/workshop
The core element recommended for the diagnostic process is a structured roundtable discussion or workshop. This format is well suited for the purpose of bringing the different groups of stakeholders together with different perspectives, background and knowledge. The goal is to identify problems and possible solutions together.
The EMCDDA has piloted this approach in Luxembourg and Poland.
A manual to guide people through the process of conducting this type of diagnostic process, including the lessons learned from conducting the exercise in these countries will be launched towards the end of 2019. The manual will include the in-depth description of the preparatory process, resources needed, and also provide examples of the objectives for a roundtable discussion.
Spotlight: Luxembourg
The first pilot roundtable discussion took place on 22 January in the Directorate of Health in Luxembourg. It was organised jointly by the staff of the national Reitox Focal Point and experts from the Luxembourg Institute of Health (LIH).
Why did they have this event?
In Luxembourg, hepatitis C antibodies are present among approximately 75% of all PWID, which may in the near future result in high morbidity and mortality due to hepatitis C. The National Action Plan against Hepatitis outlines a decrease of the prevalence rate as a priority for the country. The purpose of the roundtable was to bring together an interdisciplinary group of experts working in different areas related to HCV testing and access to care for PWID to discuss how to improve HCV testing and health care practices and to empower drug treatment centres to facilitate access to care. The goal was to achieve a common understanding, a consensus, about the main barriers to HCV screening and access to treatment among policy makers and service providers, taking the reality of the clients’ situation into account, and to propose a list of actions, reflecting the priorities and their feasibility.
Who was involved?
The roundtable gathered 21 national health professionals, as well as experts from the EMCDDA and the Robert Koch Institute. The participants were the representatives of the Department of Penitentiary Psychiatric Medicine, specialists from the National Service of Infectious Diseases, nurses, social workers, directors of drug treatment and harm reduction centres, social workers and educators, and other stakeholders.
What was achieved?
The initiative allowed fruitful discussions on the main perceived barriers as well as identifying priority areas for action regarding hepatitis C testing and access to treatment for PWID. The meeting resulted in a report which systematically documents the barriers to testing on policy-, provider- and client levels in Luxembourg as well as opportunities and possible solutions. The feasibility and prioritisation of the individual measures was discussed, linking them to Luxembourg’s Hepatitis Action Plan 2018-2022 which follows the global agenda to eliminate viral hepatitis as a public health threat by 2030.
Participants found the process useful and particularly appreciated the opportunity to work together with many different stakeholders sharing their experiences:
- ‘I can guide people better, because I know how the different services work.’
- ‘The same kind of meeting should be organized for other relevant problems in the drug scene: quality design in harm reduction; working together between the drug working organization and the police.'
- 'We all agree that we need national guidelines and political support.'

Roundtable participants appreciated the fact that core experts were gathered and that all stakeholders were working together, developing a network for the response to viral hepatitis. The meeting allowed them to get to know the other service providers, their actors and their functioning; to learn about other points of view and to share experiences. Key-messages from the evaluation survey indicated several key points, such as: the importance of working together, bringing the issue to the political level, the need for improvements in access to other health services and housing, the need to develop common guidelines, the importance of staff motivation in treatment centers to improve the situation and work in the same direction. Respondents also suggested inviting other experts as well as political representatives, the city of Luxembourg, and (former) drug users to any further meetings of this kind.
Spotlight: Poland
The second pilot discussion/workshop was organised by Polish National Bureau for Drug Prevention and the National Public Health Institute and supported by staff from the EMCDDA. It took place on 11th of June 2019 in Poland.
Why did they have this event?
A recent national seroprevalence study among PWID in Poland detected that more than half of the population had been infected with HCV. A national hepatitis policy document does not yet exist, but under the national Drug Prevention Bureau two initiatives (both in Warsaw) have recently been launched to improve HCV testing among PWID. The goals of the roundtable discussion were to improve the understanding of barriers and facilitators for HCV testing in Poland and to improve the access and availability of health care in the area of HCV.
Who was involved?
There were 25 participants with diverse backgrounds, such as medical doctors, representatives of National Bureau of Drug Prevention, EMCDDA, Health Ministry, Harm reduction workers, drug services workers, the representatives of Public Health Institute, Medical University, Association of Epidemiologists and Hepatologists, as well as local government representatives and other stakeholders.
What was achieved?
The participants worked together towards a consensus regarding main barriers for HCV testing and health care delivery. They created a list of possible actions to overcome or diminish these barriers and prioritised them in terms of feasibility.
The participants reported finding the debate very valuable, providing an opportunity to share information, knowledge, opinion and experiences between many different stakeholders:
- ‘Gathering all barriers and recommendations together and defining specific actions to implement these recommendations was very useful’
- ‘We should maintain the … Regularity of meetings for the assessment, evaluation and summary of progress of actions aimed at solving the problem’
- ‘The meeting had high professional level, a large dose of practical information, as well as many different approaches and solutions to identified problems’.

Participants found the roundtable very useful and well-organised. The meeting gave them the opportunity to deepen their understanding of the situation regarding Hepatitis situation in Poland and in other EU countries. In the evaluation survey they mentioned that the level of the presentations and discussion was very professional with a good dosage of high-quality data. They could discuss different point of views, exchange experiences and work together with national experts and health professionals. The key messages of the meeting were: the importance of establishing the common ground, the need of eliminating barriers in HCV testing, the collection of the recommendations for future action, the importance of facilitating the PWID in their access to treatment, and the plans for creation of the national standards of Harm Reduction and National Hepatitis Strategy. Respondents also suggested inviting other experts, such as representatives of National Health Fund, staff of opioid substitution programmes and staff of addiction treatment services. They mentioned that it is important to repeat these meetings regularly to follow up, monitor and evaluate the actions undertaken to solve the problem.
The report from the meeting is under preparation by National Bureau for Drug Prevention.
Selecting appropriate responses
Selecting appropriate responses: models of care for increasing PWID’s access to HCV testing and treatment through drugs services
Available data shows that improving testing and linkage to care for people who inject drugs (PWID) is a central requirement for reaching the elimination target in Europe. Testing is among the most important interventions to prevent infections and European guidance recommends routine, voluntary testing as part of a regular annual health examination.
To offer testing in places where PWID are already in contact with the health care system, for example in drugs services, is more likely to be successful and part of ensuring a people-centred approach to health. In this sense, drug treatment and harm reduction services are an ideal starting point for micro-elimination of hepatitis C in PWID. This setting not only provides an opportunity to identify chronic carriers through active screening, but can also be a place where further diagnostics, linkage to care and even treatment of hepatitis C can be made more easily accessible to this important target group. Another advantage of the setting is the availability of qualified staff, including peer workers, who can relate to PWID and organise and support an efficient chain of care. Finally, drug treatment is firmly integrated in national drug-related monitoring structures, which facilitates data gathering on the hepatitis burden and trends. However, several barriers may exist which prevent testing from being implemented in drugs services.
To promote the dissemination of good implementation practices in the field of hepatitis C testing and care for PWID, a collection of case studies has been compiled. These case studies illustrate how drug treatment and harm reduction service providers in eight countries are aiming to increase access to testing and treatment among their clients, using innovative and creative implementation practices and thus developing new models of care. The publication provides key insights into the results, impact, sustainability and transferability of each practice to guide the implementation of these new models of care in other countries and settings.
The summaries of the case studies can be found on the Hepatitis C models of care topic overview page and more detailed information on each case study in the full report in the Documents Library.
Material to support action
Materials to support action
The EMCDDA Knowledge Questionnaire (KQ): Refreshing knowledge and understanding training needs
There have been great improvements in both hepatitis treatment and testing methods that provide the opportunity for transforming hepatitis C care. However, the literature shows that knowledge about the new hepatitis treatment and testing methods among staff working in harm reduction settings is not always sufficient or up to date. This may have implications for the clients, who may not be informed about new developments in this area and as a result may be less willing to be tested and/or treated.

Well-informed staff is seen as the cornerstone for action to increase testing and treatment (see Figure). The WHO European Hepatitis Action Plan calls for continuous education of healthcare professionals on viral hepatitis testing and diagnosis-related issues and defines a milestone for 2018 of having all health care workers aware of their HBV and HCV status.
Building on experiences from the DRUCK study (Zimmermann et al., 2019), a Knowledge Questionnaire was developed.
The main aims of the Knowledge Questionnaire are:
- to refresh knowledge on HCV and HBV transmission, testing and care for PWID among those working in drug treatment settings;
- to increase awareness of staff of the importance of knowing their own status;
- to provide a tool for managers of drug treatment facilities, or other actors, who would like to identify the training needs of staff.
The Knowledge Questionnaire covers a wide range of aspects regarding viral hepatitis B and C, such as prevalence, routes of transmission, prevention measures, treatment and testing options, and international recommendations on testing and treatment.
It consists of a series of true statements which provide factual information on viral hepatitis and it can be administered as an online survey or on paper. The answer format ‘I knew it already’ or ‘This is new to me’ allows participants to assess their own level of knowledge and refresh it, which facilitates an honest self assessment, avoiding a test situation with true or false statements, that may feel threatening.
Experiences with the questionnaire during the piloting phase suggest that it can be answered individually or in discussions in a small group, and that it can have a number of other uses, for example supporting better informed discussions with policymakers or to structure a dialogue between staff and clients at drugs services.
- ‘This questionnaire could be also useful for the staff to discuss the subject of HCV with their clients (sort of checklist).’
- ‘We prefer the current version of questionnaire (I heard about it/This is new to me) over true and false – to avoid putting pressure on the staff.’
Feedback from about 150 people working in drugs services in four countries who piloted the questionnaire showed that 90% of respondents found the questionnaire interesting, and 76% said that they learned something new. The majority of respondents agreed that the answer format facilitates staff to refresh their knowledge and thought people would answer the questionnaire honestly.
The knowledge questionnaire is availalbe in a variety of languages and you can download the latest version in our Document Library.
Resources
Policy
Epidemiology
Recommendations for practice
- ECDC Public health guidance on HIV, hepatitis B and C testing in the EU/EEA
- WHO Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection (2018)
- WHO Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection (2015)
- WHO Guidelines on hepatitis B and C testing (2017)
- WHO Global health sector strategy on viral hepatitis 2016-2021
- EASL Recommendations on Treatment of Hepatitis C 2018
- EACS guidelines
EMCDDA publications and resources
- Hepatitis C among drug users in Europe: epidemiology, treatment and prevention
- Drug-related infectious diseases in Europe: update from the EMCDDA expert network (May 2020)
- EMCDDA meeting on the key indicator on Drug-related Infectious Diseases (DRID) 15-16 June 2015, Lisbon
- EMCDDA knowledge questionnaire on viral hepatitis for drug service staff
Examples of promotional materials
Some examples of promotional material from other hepatitis C initiatives may be found below.






















