Heroin drug profile
Heroin drug profile
Heroin is a crude preparation of diamorphine. It is a semisynthetic product obtained by acetylation of morphine, which occurs as a natural product in opium: the dried latex of certain poppy species (e.g. Papaver somniferum L.). Diamorphine is a narcotic analgesic used in the treatment of severe pain. Illicit heroin may be smoked or solubilised with a weak acid and injected. Whereas opium has been smoked since historical times, diamorphine was first synthesised in the late nineteenth century. Heroin is under international control.
Chemistry
Molecular structure (1)
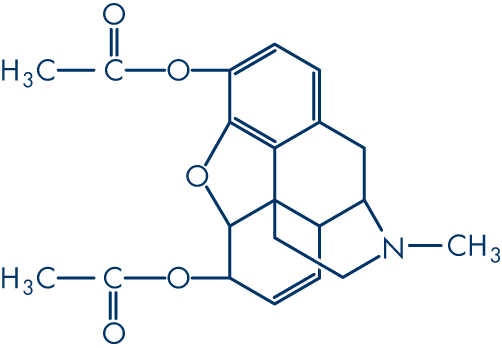
Molecular formula: C21H23NO5
Molecular weight: 369.4 g/mol
Diamorphine (diacetylmorphine; CAS-561-27-3) is produced by the acetylation of crude morphine. The systematic name (IUPAC) is (5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol acetate. Although five pairs of enantiomers are theoretically possible in morphine, only one occurs naturally (5R, 6S, 9R, 13S, 14R).
(1) Diacetylmorphine: the principal psychoactive constituent of heroin
Physical form
South-west Asian heroin is a brown powder usually in the form of the free base, which is insoluble in water but soluble in organic solvents. The less common south-east Asian heroin is usually a white powder in the form of the hydrate hydrochloride salt (CAS-1502-95-0), which is soluble in water but insoluble in organic solvents.
Pharmacology
Diamorphine, like morphine and many other opioids, produces analgesia. It behaves as an agonist at a complex group of receptors (the μ, κ and δ subtypes) that are normally acted upon by endogenous peptides known as endorphins. Apart from analgesia, diamorphine produces drowsiness, euphoria and a sense of detachment. Negative effects include respiratory depression, nausea and vomiting, decreased motility in the gastrointestinal tract, suppression of the cough reflex and hypothermia. Tolerance and physical dependence occur on repeated use. Cessation of use in tolerant subjects leads to characteristic withdrawal symptoms. Subjective effects following injection are known as ‘the rush’ and are associated with feelings of warmth and pleasure, followed by a longer period of sedation. Diamorphine is 2–3 times more potent than morphine. The estimated minimum lethal dose is 200 mg, but addicts may be able to tolerate ten times as much. Following injection, diamorphine crosses the blood–brain barrier within 20 seconds, with almost 70 % of the dose reaching the brain. It is difficult to detect in blood because of rapid hydrolysis to 6-monoacetylmorphine and slower conversion to morphine, the main active metabolite. The plasma half-life of diamorphine is about three minutes. Morphine is excreted in the urine largely as the glucuronide conjugate. Diamorphine is associated with far more accidental overdoses and fatal poisonings than any other scheduled substance. Much morbidity is caused by infectious agents transmitted by unhygienic injection.
Origin/extraction
The latex from the seed capsules of the opium poppy (Papaver somniferum L.) is allowed to dry. This material (opium) is dispersed in an aqueous solution of calcium hydroxide (slaked lime). The alkalinity is adjusted by adding ammonium chloride, causing morphine base to precipitate. The separated morphine is boiled with acetic anhydride. Sodium carbonate is added, causing the crude diamorphine base to separate. Depending on the region, this may be used directly, further purified or converted into the hydrochloride salt.
Until the late 1970s, nearly all heroin consumed in Europe came from south-east Asia, but now most originates from south-west Asia, an area centred on Afghanistan and Pakistan. Heroin is also produced in certain parts of South America, but that material is rarely seen in Europe. Acetic anhydride, an essential precursor in the manufacture of heroin, is listed in Table I of the United Nations 1988 Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. The corresponding EU legislation is set out in Council Regulation (EEC) No 3677/90 (as later amended), which governs trade between the EU and third countries. As with other naturally occurring drugs of misuse, total synthesis of the active principles is not currently an economic proposition.
Mode of use
Heroin from south-west Asia may be ‘smoked’ by heating the solid on a metal foil above a small flame and inhaling the vapour. Those intending to inject this form of heroin must first solubilise it with, for example, citric acid or ascorbic acid. Heroin from south-east Asia is suitable for direct injection of a solution. A typical dose is 100 mg at street level purity. Except when used therapeutically as an analgesic drug, ingestion of diamorphine/heroin is a much less effective route of administration.
Other names
A large number of street terms are in use, including horse, smack, shit and brown.
Analysis
In common with many other opioids, the Marquis field test produces a violet/purple coloration. In the mass spectrum, the major ions are m/z = 327, 43, 369, 268, 310, 42, 215 and 204. Using gas chromatography, the limit of detection of both diamorphine and 6-monoacetylmorphine is 100 μg/L.
Control status
Heroin is listed in Schedule I of the United Nations 1961 Single Convention on Narcotic Drugs. Diamorphine is also included in a generic sense since the 1972 Protocol, which revised the 1961 Convention, extended control to esters and ethers of scheduled substances. Thus, diamorphine is the diacetyl ester of morphine (Schedule 1).
Medical use
Diamorphine is a narcotic analgesic with limited use in the treatment of severe pain.
Publications
Infographics and media
Bibliography
Cooper, D. A. (1989), ‘Clandestine production processes for cocaine and heroin’, in: Klein, M., Sapienza, F., McClain, H. and Khan, I. (eds.) Clandestinely produced drugs, analogues and precursors: problems and solutions, United States Department of Justice Drug Enforcement Administration, Washington, DC.
King, L. A. and McDermott, S. (2004), ‘Drugs of abuse’, in: Moffat, A. C., Osselton, M. D. and Widdop, B. (eds.), Clarke's analysis of drugs and poisons, 3rd edn, Vol. 1, pp. 37–52, Pharmaceutical Press, London.
Moffat, A. C., Osselton, M, D. and Widdop, B, (eds.) (2004), Clarke's analysis of drugs and poisons, 3rd edn, Vol. 2, Pharmaceutical Press, London.
Schiff, P. L. (2002), ‘Opium and its alkaloids’, American Journal of Pharmaceutical Education 66, pp. 186–194.
United Nations (2006), Multilingual Dictionary of Narcotic Drugs and Psychotropic Substances under International Control, United Nations, New York.
United Nations Office on Drugs and Crime (2004), World Drug Report 2004, Vol. 1: Analysis, United Nations Office on Drugs and Crime, Vienna (http://www.unodc.org/pdf/WDR_2004/volume_1.pdf).